
severe hypotension, poor kin perfusion, confusion, or low cardiac output that could not otherwise be explained) Potentially life-threatening bleeding with signs or symptoms of hemodynamic compromise (i.e.Acute Major Bleeding = bleeding having one or more of the following features:.Patients enrolled at 63 centers in North America and Europe.
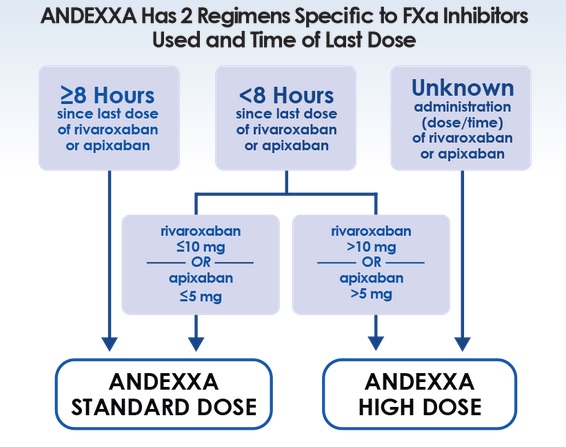
Multicenter, prospective, open-label, single-group cohort study, assessing the biological efficacy and safety of andexanet in patients with acute major bleeding.Enoxaparin, Edoxaban, or Rivaroxaban ≤7hrs prior to Andexanet Alfa bolus or unknown time = 800mg over 30 minutes followed by 2hr infusion dose of 960mg.Apixaban or rivaroxaban >7hrs prior to Andexanet Alfa bolus = 400mg over at minutes and then a 2hr infusion dose of 480mg.Patients with acute major bleeding within 18 hours after administration of a factor Xa inhibitor were given a bolus of andexanet alpha followed by a 2-hour infusion.Andexanet Alfa, a Novel Antidote to the anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4).In May of 2018, Andexanet alfa gained accelerated approval by the FDA for the reversal of these agents, but robust evidence in its support have been lacking. These agents unfortunately do not have any specific reversal agents.

Despite these advantages, the risk of bleeding remains a concern. The reason for this is the ease of use, standard dosing with no levels to check and no injections needed. Background: Over the past few years we have seen a surge in the use of oral Factor Xa inhibitors (apixaban, rivaroxaban etc) for anticoagulation.


 0 kommentar(er)
0 kommentar(er)
